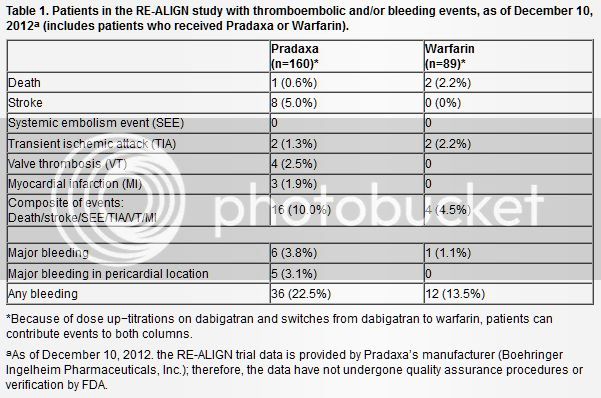
The trials weren't in the US, but Canada and several European countries. I know this was one of the drugs some were hoping would be approved to replace Coumadin for valves, so it's a shame it doesnt look like it will any time soon.
http://www.theheart.org/article/1487131.do
"Boehringer Ingelheim is halting the RE-ALIGN trial investigating the use of dabigatran (Pradaxa) in patients with artificial heart valves [1]. The phase 2 dose-ranging study was stopped because investigations into the dosing regimen "did not achieve the desired results," according to the company.
The study was testing three doses of dabigatran in patients with newly or previously implanted mechanical aortic valves, but concerns have been raised about the risk of valve thrombosis with the newer anticoagulant. In September 2012, as reported by heartwire, Canadian physicians reported the cases of two women who had undergone valve replacement years prior and had been faring well on warfarin but who subsequently suffered valve thrombosis when they were switched to dabigatran.
Dabigatran is not approved for patients with mechanical valves. It is approved by the Food and Drug Administration for preventing strokes and systemic embolism in patients with nonvalvular atrial fibrillation as well as in preventing venous thromboembolism following elective knee- or hip-replacement surgery.
"The presence of an artificial heart valve in patients is a clinical condition that is distinct from those for which dabigatran is an approved treatment," Boehringer Ingelheim stated in a press release. "In view of the interim trial results, the company is currently in discussions with the relevant regulatory authorities to reinforce the product label text accordingly and to discuss appropriate communication to physicians and relevant healthcare providers."
The RE-ALIGN study was started in December 2011, and approximately 370 patients were expected to be enrolled. The study's completion date was initially sometime in 2018"
Here is a link with info about the trial and where it was taking place http://clinicaltrials.gov/ct2/show/study/NCT01505881?term=REALIGN&rank=1&show_locs=Y#locn
http://www.theheart.org/article/1487131.do
"Boehringer Ingelheim is halting the RE-ALIGN trial investigating the use of dabigatran (Pradaxa) in patients with artificial heart valves [1]. The phase 2 dose-ranging study was stopped because investigations into the dosing regimen "did not achieve the desired results," according to the company.
The study was testing three doses of dabigatran in patients with newly or previously implanted mechanical aortic valves, but concerns have been raised about the risk of valve thrombosis with the newer anticoagulant. In September 2012, as reported by heartwire, Canadian physicians reported the cases of two women who had undergone valve replacement years prior and had been faring well on warfarin but who subsequently suffered valve thrombosis when they were switched to dabigatran.
Dabigatran is not approved for patients with mechanical valves. It is approved by the Food and Drug Administration for preventing strokes and systemic embolism in patients with nonvalvular atrial fibrillation as well as in preventing venous thromboembolism following elective knee- or hip-replacement surgery.
"The presence of an artificial heart valve in patients is a clinical condition that is distinct from those for which dabigatran is an approved treatment," Boehringer Ingelheim stated in a press release. "In view of the interim trial results, the company is currently in discussions with the relevant regulatory authorities to reinforce the product label text accordingly and to discuss appropriate communication to physicians and relevant healthcare providers."
The RE-ALIGN study was started in December 2011, and approximately 370 patients were expected to be enrolled. The study's completion date was initially sometime in 2018"
Here is a link with info about the trial and where it was taking place http://clinicaltrials.gov/ct2/show/study/NCT01505881?term=REALIGN&rank=1&show_locs=Y#locn